
Web Developers
Executive Summary
In this report, we examine some of the latest technology in healthcare and explore how this can transform the field as we know it. We will look at the following emerging technological developments in healthcare: gene editing and gene therapy, personal genomics, nanomedicine, organs-on-chips and regenerative medicine, as well as other trends, such as wearable technology and robotics. Gene editing/gene therapy: Gene editing or gene therapy involves the introduction of new genes, usually into the area of the body most affected, to replace missing or defective ones. There are some 4,000 diseases linked to genetic dysfunction, according to the American Medical Association. Personal genomics: This is the sequencing and analysis of an individual’s DNA, to identify his or her various hereditary traits and the likelihood of being prone to developing certain conditions or disorders. Conventional drugs do not work for about 75% of cancer patients, on average. Nanomedicine: Nanomedicine is the application of nanotechnology for medical use. The global nanomedicine market was worth US$248.3 billion in 2014 and is projected to grow at a CAGR of 16.3% to reach US$528.3 billion in 2019. [caption id="attachment_90817" align="aligncenter" width="368"] Source: Shutterstock[/caption]
Organs-on-chips: These are tiny laboratory-synthesized cells placed on a chip to emulate the workings of human organs. The market for organs-on-chips was worth some US$31.5 million in 2015, and growth is expected to surge at a CAGR of 70% through to 2020.
Regenerative medicine: Regenerative medicine is an interdisciplinary field that aims to restore the function and form of degenerated cells, tissues and organs to their former or original state. The regenerative medicines market is estimated to be worth some US$17.1 billion by the end of 2016, and to grow at a CAGR of 23.7% through to 2021, at which time it is expected to be worth around US$49.4 billion.
While these tech developments all look very promising, they need to be able to achieve three things to commercialize quickly and have a meaningful impact: 1) they must solve problems that patients and healthcare professionals face regularly; 2) they need to be affordable to the mass market; and 3) they need to get through the long stages of clinical trials and gain regulatory approvals faster.
Source: Shutterstock[/caption]
Organs-on-chips: These are tiny laboratory-synthesized cells placed on a chip to emulate the workings of human organs. The market for organs-on-chips was worth some US$31.5 million in 2015, and growth is expected to surge at a CAGR of 70% through to 2020.
Regenerative medicine: Regenerative medicine is an interdisciplinary field that aims to restore the function and form of degenerated cells, tissues and organs to their former or original state. The regenerative medicines market is estimated to be worth some US$17.1 billion by the end of 2016, and to grow at a CAGR of 23.7% through to 2021, at which time it is expected to be worth around US$49.4 billion.
While these tech developments all look very promising, they need to be able to achieve three things to commercialize quickly and have a meaningful impact: 1) they must solve problems that patients and healthcare professionals face regularly; 2) they need to be affordable to the mass market; and 3) they need to get through the long stages of clinical trials and gain regulatory approvals faster.
Introduction
Modern healthcare and technology have always been intertwined. From the invention of basic surgical tools such as scalpels and probes in the 1800s to the exceptionally sophisticated health equipment in use today, scientists, technologists and innovators quest for life-saving creations is never ending. In this report, we examine some of the latest technology in healthcare, and explore how this can transform the field as we know it. We will look at the following emerging developments in healthcare:- Gene editing and gene therapy
- Personal genomics
- Nanomedicine
- Organs-on-chips
- Regenerative medicine
- Other trends, such as wearable technology and robotics.
 Source: Shutterstock[/caption]
Source: Shutterstock[/caption]
DIGITAL HEALTHCARE: soon to be a US$136 billion market
Health technology, as the World Health Organization describes it, is “the application of organized knowledge and skills in the form of devices, medicines, vaccines, procedures and systems developed to solve a health problem and improve quality of lives.” Digital healthcare, a part of health technology, involves the use of information and communication systems, to address a patient’s health conditions. The global digital healthcare market was worth US$60.8 billion in 2013, according to management consultancy Arthur D. Little. The firm estimates this could increase to US$135.9 billion in 2017 and reach nearly US$233.3 billion in 2020. [caption id="attachment_90820" align="aligncenter" width="511"]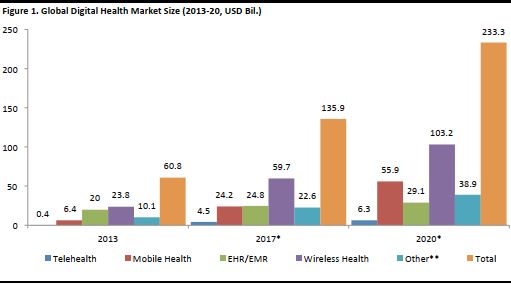 EHR is Electronic Health Records; EMR is Electronic Medical Records. Source: Arthur D. Little/GSMA Intelligence/Allied Market Research/Accenture/IHS/MarketsandMarkets [/caption]
Within the overall digital health market:
EHR is Electronic Health Records; EMR is Electronic Medical Records. Source: Arthur D. Little/GSMA Intelligence/Allied Market Research/Accenture/IHS/MarketsandMarkets [/caption]
Within the overall digital health market:
- Telehealth is expected to be the fastest-growing segment, with a CAGR of 46%. This segment comprises medical devices and communication technology to monitor patients, including video consultations with physicians, which we discussed extensively in our previous report, The Uberification of Healthcare.
- Mobile health, another promising segment, is expected to grow at a CAGR of 36%. Mobile apps and services are categorized under this segment. The wireless health segment includes handheld devices and sensors, and is expected to grow at a CAGR of 23%.
- Electronic health records (EHR) and electronic medical records (EMR) are already established segments, so these are expected to grow at a slower CAGR of 6%.
1. Gene Editing and Gene Therapy
Genes are microscopic components of human, animal or plant cells that impart hereditary characteristics to the offspring of the parent. Genes are made up of DNA and act as instructions to make proteins. Some genes may be structured differently from regular, normally-functioning genes, which cause health conditions or diseases in a living organism. [caption id="attachment_90821" align="aligncenter" width="368"] Source: Shutterstock[/caption]
Gene editing or gene therapy involves the introduction of new genes, usually into the area of the body most affected, to replace missing or defective ones.
Source: Shutterstock[/caption]
Gene editing or gene therapy involves the introduction of new genes, usually into the area of the body most affected, to replace missing or defective ones.
Why is it important?
There are some 4,000 diseases linked to genetic dysfunction, according to the American Medical Association. Some of the possible diseases that gene therapy can address are AIDS, cancer, cardiovascular disease, arthritis, and Parkinson’s and Alzheimer’s diseases. The figure below shows the conditions being addressed by clinical trials. [caption id="attachment_90822" align="aligncenter" width="380"]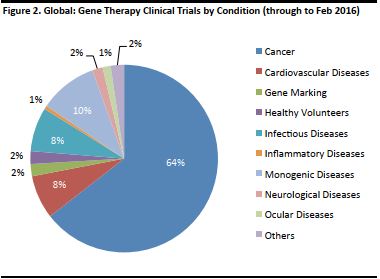 Source: The Journal of Gene Medicine, John Wiley and Sons Ltd.[/caption]
Gene editing also forms an important part of research studies. Scientists are using this method to observe the effects of altering the genes of living organisms, on their regular functions and behavior.
Source: The Journal of Gene Medicine, John Wiley and Sons Ltd.[/caption]
Gene editing also forms an important part of research studies. Scientists are using this method to observe the effects of altering the genes of living organisms, on their regular functions and behavior.
The Current State of Gene Therapy
Scientists performed the first successful gene therapy treatment in 1990. Since then, there have been many clinical trials but very few have made it to advanced stages. Clinical trials are conducted among a group of people to determine if a treatment is safe and effective. They are regulated by government and are conducted in a series of steps, called phases, with each intended to answer a separate research question. Below is a summary of the various phases in clinical trials, as outlined by the US Food and Drug Administration (FDA). [caption id="attachment_90823" align="aligncenter" width="530"]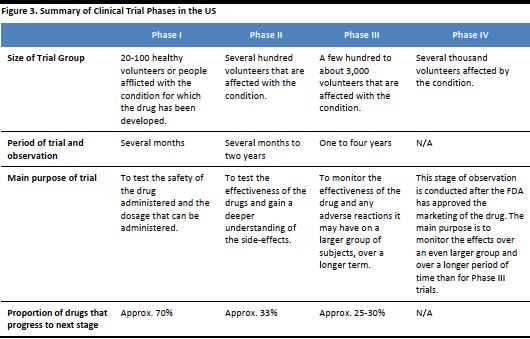 Source: US FDA[/caption]
Some drug trials may involve multiple stages, for example, one stage may be to test the highest dose that is safe, while the next stage may be to test the effectiveness of the drug. These are sometimes denoted as Phase I/II or Phase II/III. When a trial is conducted only on an individual patient, to investigate how he or she may respond to treatment and test the effectiveness of the drug, it is called a Single Subject trial.
Medicines and treatments need to pass these phases in clinical trials in order to be made available to the mass market and sell successfully. As of February 2016 (the latest date for which data is available), very few trials had reached advanced stages in the US, so we may still be a long way off before we see gene therapy used in regular clinical practice.
[caption id="attachment_90824" align="aligncenter" width="374"]
Source: US FDA[/caption]
Some drug trials may involve multiple stages, for example, one stage may be to test the highest dose that is safe, while the next stage may be to test the effectiveness of the drug. These are sometimes denoted as Phase I/II or Phase II/III. When a trial is conducted only on an individual patient, to investigate how he or she may respond to treatment and test the effectiveness of the drug, it is called a Single Subject trial.
Medicines and treatments need to pass these phases in clinical trials in order to be made available to the mass market and sell successfully. As of February 2016 (the latest date for which data is available), very few trials had reached advanced stages in the US, so we may still be a long way off before we see gene therapy used in regular clinical practice.
[caption id="attachment_90824" align="aligncenter" width="374"] Source: Shutterstock[/caption]
[caption id="attachment_90825" align="aligncenter" width="373"]
Source: Shutterstock[/caption]
[caption id="attachment_90825" align="aligncenter" width="373"]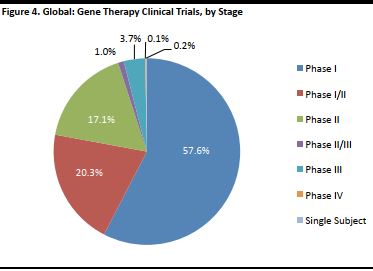 Source: The Journal of Gene Medicine, John Wiley and Sons Ltd.[/caption]
However, in 2012, the European Medicines Agency (EMA), the regulatory authority for the European Union, approved a gene therapy drug called Glybera for the treatment of lipoprotein lipase deficiency (LPLD), a rare genetic disorder. The drug formally launched in Germany in 2015 at a market price of about US$1.4 million per course of treatment, and has since been used to treat only one patient.
UniQure, the firm that developed Glybera, has not found much success in gaining regulatory approval elsewhere. And, perhaps, unsurprisingly, given the price tag, it has come under the scrutiny of cost-effectiveness watchdogs in Germany.
The EMA approved a second gene therapy drug, called Strimvelis, for children suffering with Adenosine Deaminase Severe Combined Immune Deficiency (ADA-SCID), an ultra-rare genetic disorder that on average just 15 children are born with in Europe each year. Industry giant GlaxoSmithKline, the developer of the drug, received marketing authorization in May 2016, and is selling the one-time treatment drug for US$665,000 with a money-back guarantee.
Source: The Journal of Gene Medicine, John Wiley and Sons Ltd.[/caption]
However, in 2012, the European Medicines Agency (EMA), the regulatory authority for the European Union, approved a gene therapy drug called Glybera for the treatment of lipoprotein lipase deficiency (LPLD), a rare genetic disorder. The drug formally launched in Germany in 2015 at a market price of about US$1.4 million per course of treatment, and has since been used to treat only one patient.
UniQure, the firm that developed Glybera, has not found much success in gaining regulatory approval elsewhere. And, perhaps, unsurprisingly, given the price tag, it has come under the scrutiny of cost-effectiveness watchdogs in Germany.
The EMA approved a second gene therapy drug, called Strimvelis, for children suffering with Adenosine Deaminase Severe Combined Immune Deficiency (ADA-SCID), an ultra-rare genetic disorder that on average just 15 children are born with in Europe each year. Industry giant GlaxoSmithKline, the developer of the drug, received marketing authorization in May 2016, and is selling the one-time treatment drug for US$665,000 with a money-back guarantee.
The Outlook
Aside from UniQure and GlaxoSmithKline, which appear to be the only companies that have so far received regulatory approval for gene therapy treatments, there is no clear market leader yet. New entrants face the hurdle of overcoming the high price tag the drugs are likely to carry.2. Personal Genomics
Personal genomics is the sequencing and analysis of an individual’s DNA, to identify his or her various hereditary traits and likelihood of being prone to developing certain conditions or disorders.Why is it Important?
Genes hold a wealth of information about people’s unique characteristics and their lineage. A person’s genes may be able to provide an indication of common physical and psychological traits associated with ancestry, the health conditions and illnesses that run in the family, and the things one should do to stay healthy. Personalized medicine, a branch of medicine that treats people based on their genetic sequence, aims to increase the accuracy of diagnosis and treatment for some conditions, as the drugs typically used for the condition may be ineffective in treating them. Conditions such as cancer are, to a large extent, unique to a patient’s genetic makeup. Conventional drugs do not work for about 75% of patients, on average, according to a research paper published in the journal Trends in Molecular Medicine, which analyzes the responses of patients to drugs, based on their genetics. By knowing how a patient will respond to certain drugs based on their genetics beforehand, through personalized medicine, doctors will be able to diagnose and treat the illness more effectively. They will not have to prescribe the typical medication to the patient based on his or her health history and conditions, and then find an alternative treatment should there be an adverse reaction. Moreover, personalized medicine can enable a preventive approach to healthcare by identifying conditions a person is susceptible to, through genome tests. The person may then be able to make healthy lifestyle changes or take a course of action, such as treatment, in advance, to avoid the condition altogether. [caption id="attachment_90826" align="aligncenter" width="379"]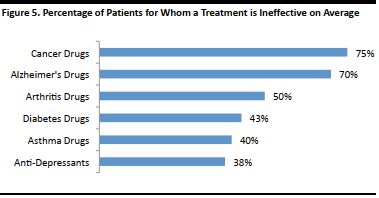 Source: Brian B. Spear, Margo Heath-Chiozzi, Jeffrey Huff, Clinical Trends in Molecular Medicine[/caption]
Source: Brian B. Spear, Margo Heath-Chiozzi, Jeffrey Huff, Clinical Trends in Molecular Medicine[/caption]
The Current State of Personal Genomics
Technological advancements in genome research have grown at a faster pace than what was declared by Moore’s Law (an idea advocated by Intel co-founder Gordon Moore that the capacity of computer processors doubles every two years). In its nascent years during the early 2000s, the cost of sequencing a human genome was around US$300 million, but now it has become far more accessible to the general public and can be done for as little as US$199 for a basic direct-to-consumer test. The table below shows the dramatic fall in the price of a single genome sequence, from 2001 to 2015. [caption id="attachment_90827" align="aligncenter" width="376"]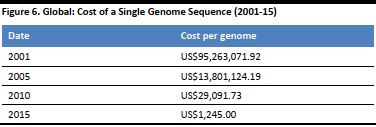 Source: National Human Genome Research Institute[/caption]
At the exorbitant costs in its early days, genome sequencing was available only for research or to the ultra-wealthy, but the drastic fall in price, due to technological advancement, has led to several direct-to-consumer companies forming as a result.
We have listed some of the popular genomics profiling companies in the following table, to provide examples of the services they provide and the approximate cost.
[caption id="attachment_90828" align="aligncenter" width="528"]
Source: National Human Genome Research Institute[/caption]
At the exorbitant costs in its early days, genome sequencing was available only for research or to the ultra-wealthy, but the drastic fall in price, due to technological advancement, has led to several direct-to-consumer companies forming as a result.
We have listed some of the popular genomics profiling companies in the following table, to provide examples of the services they provide and the approximate cost.
[caption id="attachment_90828" align="aligncenter" width="528"]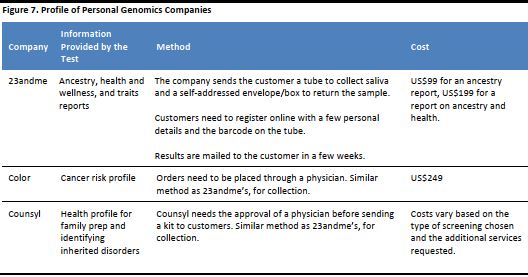 Source: Company reports[/caption]
There are several other companies that provide services similar to the ones above, with varying price ranges. There are even some that provide more specific information on a health condition and how a patient may be treated.
Source: Company reports[/caption]
There are several other companies that provide services similar to the ones above, with varying price ranges. There are even some that provide more specific information on a health condition and how a patient may be treated.
The Outlook
While many find such in-depth analysis of their genetic profile fascinating and helpful, there are others who feel that it is unnecessary to know little information without adequate detail and without the knowledge to understand it. For example, knowing that one’s DNA is a likely carrier of a disease or that one’s DNA makes him or her susceptible to a certain kind of illness may cause unwarranted worry and panic, as an average person with no medical knowledge may not understand the implications of the information given to them.3. Nanomedicine
Nanomedicine is the application of nanotechnology for medical use. Nanotechnology involves the engineering and use of miniature machines—ones that are at an atomic or molecular level. In more precise terms, the “nano-” prefix is used to signify a measure equivalent to one billionth. Nanoparticles are invisible to the human eye. [caption id="attachment_90829" align="aligncenter" width="371"] Source: Shutterstock[/caption]
Source: Shutterstock[/caption]
Why is it Important?
Experts view nanomedicine as a possible solution to the problems posed by traditional methods of drug delivery, i.e. the passage of a drug through a patient’s body to safely and effectively deliver the desired effect. In many cases, a drug may not be effectively absorbed by the body and may even be eliminated from the body before it can achieve the intended purpose. Nanotechnology allows greater control over the path a drug takes once it is ingested. The drug is placed in a carrier or a nanoparticle that is made of material suitable to attach easily to the affected area or organ. Once it reaches the right destination, the drug is dispensed from the carrier. Another application for nanomedicine is to visualize and detect tumors from MRI scans. With this method, the patient is injected with nanoparticles and an MRI scan is done. If the area of the body has a tumor, the nanoparticles will not be absorbed and show up as bright spots, helping doctors to detect the presence of a tumor. A newer application that is being tested for nanotechnology is the delivery of cancer drugs through nanorobots, or nanobots as they are commonly called. Conventional cancer treatments such as radiation or chemotherapy do not just destroy the cancerous cells, but affect healthy cells as well. On the other hand, nanobots will actively seek out cancer cells and release drugs only when they reach those cells. [caption id="attachment_90830" align="aligncenter" width="372"] Source: Shutterstock[/caption]
Source: Shutterstock[/caption]
The Current State of Nanomedicine
The first nano-drug was approved by the Food and Drug Authority (FDA) in the US in 1995, and since then, several more have been approved by the American regulatory body and a few by the Japanese and European regulatory bodies as well. This number continues to grow as doctors, scientists and researchers formulate additional methods to treat existing and new conditions. The global nanomedicine market was worth US$248.3 billion in 2014, and is projected to grow at a CAGR of 16.3% to reach US$528.3 billion in 2019, according to market-research firm Research and Markets. The market for nanomedicines for cancer was worth some US$75.4 billion in 2014, and is expected to grow at a CAGR of 13.2% through to 2019, when it will be worth about US$140.2 billion, the firm said. The next largest sub-market is for nanomedicines that address conditions related to the central nervous system. Research and Markets estimated this to be US$50.3 billion in 2014, and to grow at a CAGR of 15.4% to US$102.9 billion in 2019. [caption id="attachment_90831" align="aligncenter" width="534"]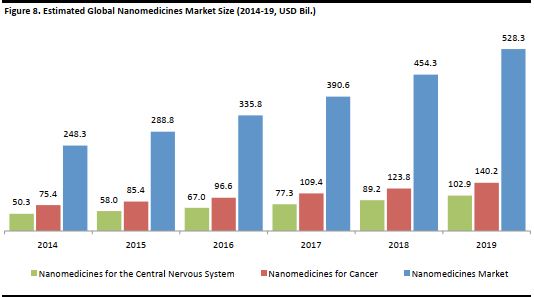 Source: Research and Markets[/caption]
According to a 2012 medical research paper, the majority of nanomedicines developed up to 2011 were for cancer, with a larger proportion in an investigative stage, while the rest were in commercial use. The figure below depicts an approximate number of nanomedicine products developed by the condition to be treated, as they were listed on the US clinicaltrials.gov database at the time of research.
[caption id="attachment_90832" align="aligncenter" width="376"]
Source: Research and Markets[/caption]
According to a 2012 medical research paper, the majority of nanomedicines developed up to 2011 were for cancer, with a larger proportion in an investigative stage, while the rest were in commercial use. The figure below depicts an approximate number of nanomedicine products developed by the condition to be treated, as they were listed on the US clinicaltrials.gov database at the time of research.
[caption id="attachment_90832" align="aligncenter" width="376"]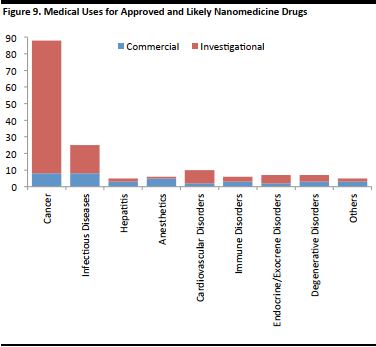 *Approximate numbers in 2012.
*Approximate numbers in 2012.Source: The Big Picture on Nanomedicine: The State of Investigational and Approved Nanomedicine Products, Elsevier [/caption]
The Outlook
Nanobots and particles for MRI imaging continue to be in stages of research and clinical trials, and getting them past regulatory approval is still a complex task. Gaining approval for medicines is in itself an arduous process, and to obtain it for the technology and robots may be a different matter altogether. The technology also needs to be refined to a level that it can be automated and relied on to the extent that it will not fail, as having machines suspended within a human body, with no real purpose could have serious implications. And while some of this technology is being developed with the idea of treating a particular condition, there are other likely treatments being developed at a faster pace that might render the former redundant. However, with the 2016 Nobel Prize in chemistry awarded to three scientists “for the design and synthesis of molecular machines,” nanotechnology and its various applications in the fields of medicine and health are likely to draw further research and interest in the near future.4. Organs-On-Chips
Organs-on-chips are tiny laboratory-synthesized cells placed on a chip to emulate the workings of human organs. This invention involves a multi-disciplinary field called microfluidics that unites engineering, physics, chemistry, biology and nanotechnology. Components of these chips are small, but not all as minute as nanoparticles, and they also serve a different function. We therefore consider them to be a separate segment. [caption id="attachment_90833" align="aligncenter" width="371"] Source: Shutterstock[/caption]
Source: Shutterstock[/caption]
Why is it Important?
Organs-on-chips facilitate a wider application of pre-clinical drug trials that normally employ animal subjects in their study, and are not a treatment in themselves. Many times the drugs may not have the same effect on humans as they do on animals, because the toxicity tolerance is different for both. Synthetic organs that function, behave and respond just as human organs do can provide more insightful observations from drug trials. An advantage that the chip-sized organs have over large ones is that they are less complex, with fewer variables to control, require less hardware support and technician time to keep them alive, and are thus more convenient to run tests on. Creating a larger organ model involves synthesizing more cells to build it, and will require a higher quantity of drugs to run tests, which ultimately may not be cost-effective. Chip organ technology can help researchers progress faster with the development of medicines and vaccines, and foster cheaper and efficient pharmaceutical, drug and cosmetic testing.The Current State of Organs-On-Chips
Harvard University’s Wyss Institute for Biologically Inspired Engineering has been a pioneer in chip organ technology, and has so far developed lungs, hearts and intestines on chips. It aims to create ten different human organs on chips and link them together through a programmed apparatus that is meant to mimic a fully functional human body. The Museum of Modern Art, New York, and the London Design Museum have even awarded Wyss Institute’s organ-on-a-chip with “Design of The Year” in a move to curb animal testing and promote more ethical drug and cosmetics trials. [caption id="attachment_90834" align="aligncenter" width="370"] Source: Shutterstock[/caption]
Pharma company AstraZeneca alone used about 182,000 animals for global research in 2015, and this is down from the 408,000 it used in 2010. Many others have also been taking action to reduce the number of animals used for testing, due to animal welfare groups prompting for hard regulation against it. Chip organ technology can turn out to be a suitable alternative should harder laws or consumer sentiment make animal testing tougher.
Source: Shutterstock[/caption]
Pharma company AstraZeneca alone used about 182,000 animals for global research in 2015, and this is down from the 408,000 it used in 2010. Many others have also been taking action to reduce the number of animals used for testing, due to animal welfare groups prompting for hard regulation against it. Chip organ technology can turn out to be a suitable alternative should harder laws or consumer sentiment make animal testing tougher.
The Outlook
Research and Markets estimated the market for organs-on-chips to be worth some US$31.5 million in 2015, and expects growth to surge at a CAGR of 70% through to 2020. As this technology is still very new and also since there are only a handful of companies (some of which are university spin-outs that have agreements with pharma companies), the demand for chip organ technology is expected to grow at a rapid pace.5. Regenerative Medicine
We discussed regenerative medicine in the context of anti-aging in our sixth report in the Silvers Series; readers can find that report on our website, FungGlobalRetailTech.com. Here, we look at the broader applications of the technology. Regenerative medicine is an interdisciplinary field that aims to restore the function and form of degenerated cells, tissues and organs to their former or original state.Why is it Important?
In 2015, there were just 15,068 organ donors—they contributed to 37,910 organ transplants—while the number of Americans that needed an organ transplant was a substantial 119,926. The ability to create new cells which can then go on to form full organs that function within a human body is being viewed as a likely solution to address the acute organ shortage problem being faced globally. [caption id="attachment_90835" align="aligncenter" width="367"] Source: Shutterstock[/caption]
Tissue engineering, a sphere of regenerative medicine, involves implanting human cells into scaffolds made of biological or synthesized material, in a controlled environment. Damaged tissues and organs can be repaired with the use of these engineered tissues, which are being applied extensively in orthopedic surgery, abdominal surgery and gastrointestinal surgery.
Source: Shutterstock[/caption]
Tissue engineering, a sphere of regenerative medicine, involves implanting human cells into scaffolds made of biological or synthesized material, in a controlled environment. Damaged tissues and organs can be repaired with the use of these engineered tissues, which are being applied extensively in orthopedic surgery, abdominal surgery and gastrointestinal surgery.
The Current State of Regenerative Medicine
Research company Markets and Markets estimates the regenerative medicines market will be worth about US$17.1 billion by the end of 2016, and grow at a CAGR of 23.7% through to 2021 when it will stand at US$49.4 billion. Currently, there are several players that are making products for joint and bone reconstructive surgeries. A growing global senior population is likely to bolster the number of patients with musculoskeletal conditions, such as arthritis, for whom engineering products like bone grafts can be used. Advancements in technology have provided a boost to stem cell research and gene therapy, and many of the solutions being developed under these branches of regenerative medicine are still in the clinical trials stage.The Outlook
Technology is one aspect that can boost the progress of regenerative medicine but in many markets government regulations also play a key role in the way the field advances. For example, Japan has specific policies in place that accelerate the approval of products and treatments in regenerative medicine. [caption id="attachment_90836" align="aligncenter" width="374"] Source: Shutterstock[/caption]
Japan instituted a special forum to bring innovative companies in the field together, and formulated policies to attract foreign companies to set up shop in the country. Of course, Japan also has an aging population and is looking for ways to address problems that may arise as a result of this growing demographic. However, it gives Japan a first-mover competitive advantage in the field, and perhaps other countries can draw some inspiration from it, on the approach to develop local emerging technology.
Source: Shutterstock[/caption]
Japan instituted a special forum to bring innovative companies in the field together, and formulated policies to attract foreign companies to set up shop in the country. Of course, Japan also has an aging population and is looking for ways to address problems that may arise as a result of this growing demographic. However, it gives Japan a first-mover competitive advantage in the field, and perhaps other countries can draw some inspiration from it, on the approach to develop local emerging technology.
6. Other Trends
Apart from the major technologies listed earlier, there are several others that are already being used extensively and have almost become the “norm” in clinical practice. We briefly outline these below.Wearables and the Smart, Healthy Home
The market for smart devices has grown exponentially in the last decade, and by the end of 2016, research firm Gartner predicts that there will be at least 6.4 billion connected devices, up from 4.9 billion at the end of 2015, and expects the number to reach at least 20 billion connected devices by the end of 2020. [caption id="attachment_90837" align="aligncenter" width="369"] Source: Shutterstock[/caption]
The rise in general health and fitness consciousness has furthered the demand for wearable trackers and devices, and this is set to grow further, with big-brand technology companies, sports brands, fashion brands and the like unveiling their own versions of smart bands, watches, eyewear and smartphone applications.
But wearables have gone a step further to help track the health and safety of aging and infirm people. Sensors placed around the home are enabling seniors and people with various health conditions to live independently, by connecting smart home devices and the wearer of the smart tracker with a remote caregiver. Smart home systems can help remind a person to take their medication or to turn off the stove or assist with other simple tasks, or even call for help during an emergency.
Source: Shutterstock[/caption]
The rise in general health and fitness consciousness has furthered the demand for wearable trackers and devices, and this is set to grow further, with big-brand technology companies, sports brands, fashion brands and the like unveiling their own versions of smart bands, watches, eyewear and smartphone applications.
But wearables have gone a step further to help track the health and safety of aging and infirm people. Sensors placed around the home are enabling seniors and people with various health conditions to live independently, by connecting smart home devices and the wearer of the smart tracker with a remote caregiver. Smart home systems can help remind a person to take their medication or to turn off the stove or assist with other simple tasks, or even call for help during an emergency.
The Rise of the “-bles”—Embeddables, Ingestibles and Hearables
Health trackers are not just wearable anymore, but can also be embedded into the skin, swallowed through a smart pill or even placed in the ear through smart headphones. These are expected to track vital health information more discreetly and even deliver results more efficiently, as physicians will have more knowledge of patient information and control the way conditions are treated.AI and VR in the OT
As technology advances, so does the alphabet soup of innovations, but abbreviations aside, artificial intelligence (AI) and virtual reality (VR) are playing substantial roles in the operating theatre (OT) and assisting surgeons with procedures. Watson, the cognitive AI from tech firm IBM, is able to read a patient’s symptoms and sift through heaps of data from clinical studies, medical books, patient records and other information, to help doctors diagnose their patients with seemingly rare or complicated conditions. [caption id="attachment_90838" align="aligncenter" width="366"] Source: Shutterstock[/caption]
VR is helping physicians and surgeons look at organs and areas of the body in more detail than ever before, which can help them deliver better results with treatments they dispense and procedures they carry out.
Source: Shutterstock[/caption]
VR is helping physicians and surgeons look at organs and areas of the body in more detail than ever before, which can help them deliver better results with treatments they dispense and procedures they carry out.